teaser
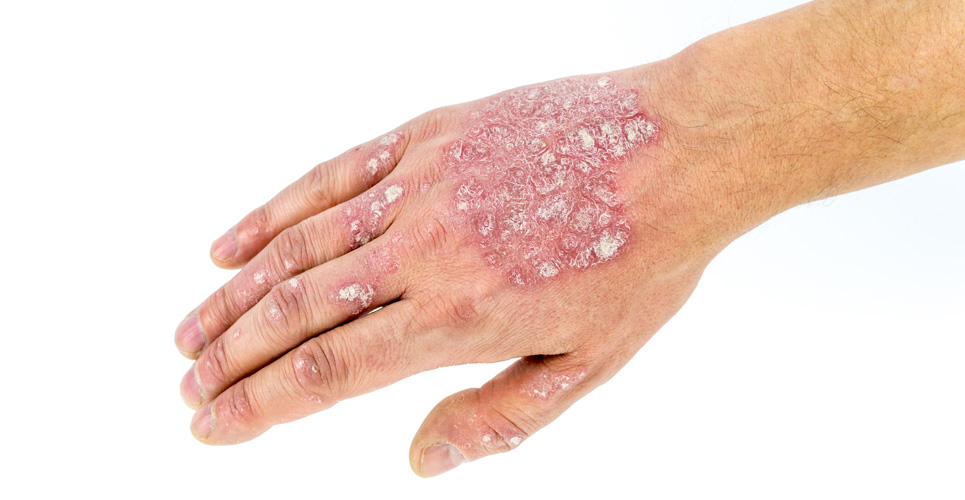
The tumour necrosis factor antagonist etanercept is highly effective in treating moderate to severe plaque psoriasis in children and adolescents, a controlled trial has shown.
Etanercept is known to be effective in plaque psoriasis in adults, but data on use in children are limited.
This trial, just published in the New England Journal of Medicine, examined the drug’s effectiveness and safety in patients aged 4-17.
Participants were children and adolescents with stable moderate to severe plaque psoriasis, defined as a psoriasis area-and-severity index (PASI) score of 12 or greater (PASI scores range from 0 to 72, higher is worse).
Other criteria included involvement of at least 10% body surface area, at least six months’ history and treatment with systemic or phototherapy or inadequate response to topical therapy.
Participants were randomised to treatment with etanercept or placebo for 12 weeks, followed by open-label etanercept for 24 weeks, then re-randomisation to etanercept or placebo for 12 weeks to investigate the effects of withdrawal and re-treatment.
During the first period, open-label escape etanercept was allowed if PASI score increased by more than 50%.
Primary outcome was 75% or greater reduction in PASI score (PASI-75) at week 12; safety endpoints included adverse effects, serious adverse effects, infections and disease rebound during the withdrawal period.
In total, 264 patients were screened, of whom 211 were randomised to treatment (etanercept n=106, placebo n=105).

Median age was 13, and 36% were aged 11 or under at randomisation; median PASI score at randomisation was 16.4 (range 12-56.7).
During the first phase (primary endpoint), 57% of patients in the etanercept group reached PASI-75 compared with 11% of the placebo group (p < 0.001).
More patients receiving placebo entered the escape group (27 vs five).
There were more adverse effects reported overall during etanercept treatment, however, because exposure periods differed significantly.
Adjusted event rates were similar for the two groups.
Most adverse events were considered to be of mild or moderate intensity: there were more serious adverse effects in patients receiving etanercept (four in three patients vs 0; all resolved on withdrawal without sequelae), including three infections, and more events were classed as severe (seven vs three).
Take part in our prize-draw survey